Abstract
Background: A major cause of mortality in patients receiving hematopoietic stem cell transplantation (HCT) is acute graft-versus-host disease (GVHD), a multiorgan disorder that includes the skin, liver and gastrointestinal tract. We have previously identified elafin, a protease inhibitor overexpressed in inflamed epidermis, as a diagnostic biomarker of GVHD in the skin, the most commonly involved GVHD organ. However, our initial study was limited to a subset of patients with isolated skin GVHD. The main driver of nonrelapse mortality (NRM) in HCT patients is GI GVHD. Two biomarkers, Regenerating islet-derived 3a (REG3α) and Suppressor of tumorigenesis 2 (ST2), have since been validated as biomarkers of GI GVHD that predict long-term outcomes in patients treated for GVHD. We undertook this study to determine the utility of elafin as a prognostic biomarker of acute GVHD in the general population of previously unstudied acute GVHD patients, and to compare it to ST2 and REG3α.
Study Design: 526 patients who received systemic corticosteroid treatment for skin GVHD were analyzed from the Mount Sinai Acute GVHD International Consortium (MAGIC), which includes patients from 25 HCT centers. We used ELISA to measure serum concentrations of elafin, ST2 and REG3α. Patients were divided randomly into equal training and validation sets; and we developed a competing risk regression model for 6-month NRM using elafin concentration in the training set. We developed additional models for 6-month NRM using concentrations of ST2 and REG3α, or the combination of all three biomarkers as predictors. We then constructed ROC curves to evaluate the predictive accuracy of each model and to analyze the ability of each model to stratify patients into high- and low-risk groups. We analyzed the cumulative incidence of 6-month NRM and overall survival in each model and compared the accuracy of each model in the validation set.
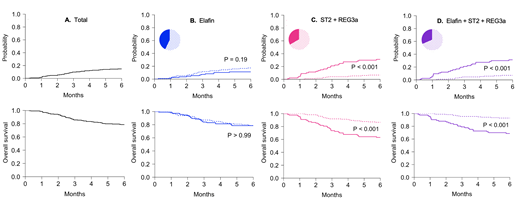
Results: The area under the receiver operating curve (AUROC) for elafin alone was 0.55 whereas it was 0.75 and statistically superior (P = 0.02) for the combination of ST2 and REG3α. The combination of 3 biomarkers produced an AUROC of 0.76 that was not significantly better than the two biomarker model (P = 0.10). Elafin concentrations, either alone or in combination with ST2 and REG3α, did not produce higher hazard ratios of NRM (data not shown). Patients in the low-risk elafin group paradoxically demonstrated a higher incidence of 6-month NRM, although this difference was not statistically significant (17% vs. 11%, P=0.19), and both overall survival at 6 months (68% vs. 68%, P>0.99) and four-week response (78% vs. 78%, P=0.98) were similar in the low- and high-risk elafin groups (Figure 1). As demonstrated in previous data sets, the combination of ST2 and REG3α divided patients into two groups with a nearly five-fold difference in NRM (6.7% vs. 31%, P <0.001).
Conclusion: We demonstrated that serum elafin concentrations measured at the initiation of systemic treatment for acute GVHD in a multicenter population of patients treated systemically for acute GVHD do not predict 6-month NRM, overall survival, or treatment response. As seen in previous studies, serum concentrations of the GI GVHD biomarkers ST2 and REG3α were significant predictors of NRM and the addition of elafin levels did not improve their accuracy. These results underscore the importance of GI disease in driving NRM in patients who develop acute GVHD.
Figure 1. Cumulative incidence of nonrelapse mortality and overall survival in high and low risk groups
Six-month cumulative incidences of nonrelapse mortality (NRM) in high (solid line) and low (dotted line) risk groups defined by optimized biomarker thresholds (upper panels) and six-month overall survival estimated using the Kaplan-Meier method (lower panels). (A) Cumulative incidence of NRM (14%) and overall survival (75%) in the total validation set (N=263). (B) Cumulative incidence of NRM in the low (N=150) and high (N=113) elafin group (17% vs. 11%, P=0.19). Overall survival in the low and high elafin group (68% vs. 68%, P > 0.99). (C) Cumulative incidence of NRM in the low (N=175) and high (N=88) ST2 + REG3a group (6.7 vs. 31%, P < 0.001). Overall survival in the low and high ST2 + REG3a group (77% vs. 51%, P < 0.001). (D) Cumulative incidence of NRM in the low (N=180) and high (N=83) elafin + ST2 + REG3a group (7.0 vs. 30%, P < 0.001). Overall survival in the low and high elafin + ST2 + REG3a group (79% vs. 64%, P < 0.001).
Ozbek: Viracor: Patents & Royalties: GVHD biomarker patent with royalties from Viracor. DeFilipp: Omeros, Corp.: Consultancy; Incyte Corp.: Research Funding; Regimmune Corp.: Research Funding; Syndax Pharmaceuticals, Inc: Consultancy. Grupp: Novartis, Kite, Vertex, and Servier: Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Steering committee, Research Funding; Novartis, Roche, GSK, Humanigen, CBMG, Eureka, and Janssen/JnJ: Consultancy; Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene and Cabaletta: Other: Study steering committees or scientific advisory boards. Hexner: Blueprint medicines: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tmunity Therapeutics: Research Funding; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees. Kitko: Co-investigator on two NIH grants as part of the cGVHD consortium: Research Funding; Vanderbilt University Medical Center: Current Employment; PER: Other: PER - CME educational talks about GVHD; Horizon: Membership on an entity's Board of Directors or advisory committees. Qayed: Novartis: Honoraria; Mesoblast: Honoraria; Medexus: Honoraria. Reshef: ilead, BMS, Precision, Immatics, Atara, Takeda, Shire, Pharmacyclics, Incyte: Research Funding; Bayer: Consultancy; Gilead and Novartis: Honoraria; BMS, Regeneron, TScan, Synthekine, Atara, Jasper, Bayer: Consultancy. Levine: Incyte: Consultancy, Research Funding; Viracor: Patents & Royalties: GVHD biomarker patent with royalties from Viracor; Mesoblast: Consultancy, Research Funding; Equillium Bio: Membership on an entity's Board of Directors or advisory committees; X4 Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Talaris Therapeutics: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees; Symbio: Membership on an entity's Board of Directors or advisory committees; Biogen: Research Funding; Kamada: Research Funding. Ferrara: Eurofins Viracor: Consultancy, Other: Royalties. Chen: Incyte: Consultancy; Gamida: Consultancy.